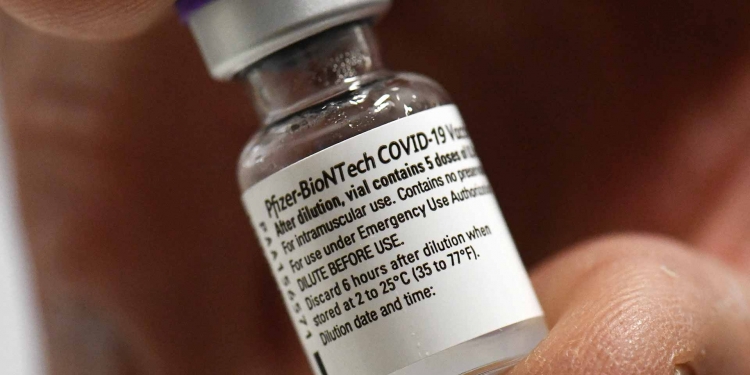
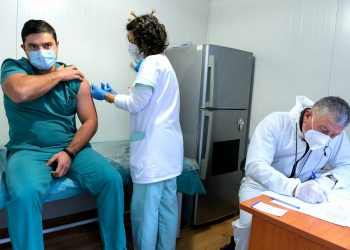
To speed up access to COVID-19 vaccinations in the developing world, on the eve of the new year, the UN health agency approved Pfizer and BioNTech’s vaccines for emergency use. Regulatory experts convened by the World Health Organization (WHO) from around the world and UN agency’s own teams reviewed the data on the Pfizer/BioNTech vaccine . They finally found last Thursday that it met WHO’s must-have criteria for safety and efficacy. Vaccines benefits are offsetting any potential risks.
“This is a very positive step towards ensuring global access to COVID-19 vaccines,” said Dr. Mariângela Simão, WHO Assistant-Director General for Access to Medicines and Health Products. But I want to emphasize the need for an even greater global effort to achieve enough vaccine supply to meet the needs of priority populations everywhere”.
Pfizer and BioNTech Celebrate Historic First Authorization in EU
Working night and day
The move opens the door for countries to expedite their own regulatory approval processes to import and administer the vaccine. It also enables UNICEF and the Pan-American Health Organization (PAHO) to procure the vaccine for distribution to countries in need. At the same time, WHO is encouraging more developers to come forward for review. Also WHO works on assessment to satisfy the critical supply for all countries globally to stem the pandemic.
WHO and our partners are working night and day to evaluate other vaccines that have reached safety and efficacy standards
Dr. Simão
Setting WHO policy
The Pfizer vaccine is also under policy review. Drawing from WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) population prioritization recommendations for COVID-19 vaccines, which were issued in September, the group will convene on 5 January to formulate vaccine specific policies and recommendations.
Meanwhile, WHO is working with regional partners to advise national health authorities about the two-dose shot and its anticipated benefits.
The World Health Organization, with the GAVI Vaccine Alliance and the Coalition for Epidemic Preparedness Innovations (CEPI), are spearheading COVAX global effort. They work to secure the equitable distribution of vaccines to all countries and not just to wealthy nations.

Pfizer – BioNTech celebrate FDA and EMA authorization
Pfizer and BioNTech announced on December 11 that the U.S. Food and Drug Administration (FDA) has authorized the emergency use of the mRNA vaccine, BNT162b2. It is effective and tested against COVID-19 for individuals 16 years of age or older. The vaccine is now authorized under an Emergency Use Authorization (EUA). Pfizer and BioNTech gather additional data. They prepare to file a planned Biologics License Application (BLA) with the FDA for a possible full regulatory approval in 2021.
Europe orders 100 million extra Pfizer vaccines
European Medicines Agency-EMA recommends first COVID-19 vaccine for authorisation. European Union orders 100 million additional doses of the Pfizer – BioNTech coronavirus vaccine. “We remain committed to moving as quickly and safely as possible to bring this vaccine to more people in Europe, as the deadly virus continues to spread at an alarming rate,” said Albert Bourla, Chairman and Chief Executive Officer, Pfizer. “In partnership with the European Commission, member states and healthcare providers, we will be able to reach a total of 150 million Europeans across the continent.”